When using the word “nutrient,” we tend to speak positively. After all, were nutrients not in those pills forced on us every morning by our mothers since before we could remember?
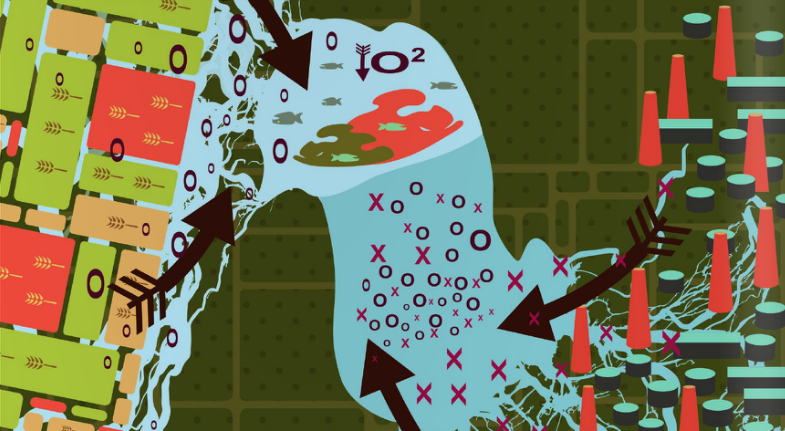
Nutrients are also needed to grow crops, and are essential components of the fertilizers that farmers spread on their land. It seems that nutrients are a very important component of society, and are required to keep us healthy and well-fed.
Unfortunately the positive way in which we talk about nutrients stops with one word: eutrophication.
So why does eutrophication have such negative connotations? Formally defined, eutrophication is the “process by which a body of water becomes enriched in dissolved nutrients.” Its roots come from two Greek words: “eu” meaning “well” and “trope,” which means “nourishment.”
“Well-nourished” does not exactly sound threatening, so we must look beyond the definition to understand the problem. We have to ask what happens when we fertilize bodies of water.
With excess nutrients such as nitrogen and phosphorus in the water, the growth of algae and plants explodes just like the growth of crops does on a farmer’s field. This leads to unsightly, sometimes foul-smelling blooms of algae.
Although quick to appear, algal blooms are not concerning, beyond their effect on the recreational value of water bodies. There are much more environmentally dangerous consequences of eutrophication, like the ones that put Lake Winnipeg in the running for the most threatened lake of the year in 2013.
Under certain conditions algal blooms can be dominated by cyanobacteria, commonly known as blue-green algae. These microorganisms produce toxins damaging to the liver, which become concentrated in the water surrounding large algal blooms.
Although many people have become ill from exposure to these toxins, human death from algal-contaminated drinking water is unlikely to occur. Wildlife and pets are at risk when they drink untreated water near a bloom of blue-green algae.
Furthermore, when algae and plants die, they sink. Once at the bottom of the lake the nutrients trapped in their cells can be released into the water column again through various biochemical processes.
This cyclic pattern of increased nutrients, algal growth and death, sedimentation, and nutrient recycle from the sediments makes solving eutrophication very difficult once the process has become established.
Another consequence of increased algal growth and death is the potential to kill off fish species. When algae die their cells begin to decay and use up oxygen.
If a large enough bloom of algae begins to die, oxygen in the water can drop below the levels required for fish to live. This is largely thought of as the point of no return, where eutrophication can no longer be suppressed or managed.
So how did we end up over-nourishing our bodies of water? Is it the agricultural industry being overzealous with the amount of fertilizer they apply? Or is urban sprawl and increased nutrient loading from cities’ wastewater discharges to blame?
The correct answer is: Yes. Without the agricultural industry our cities could not support the populations they do, and without cities the agricultural industry could not support the work they do. No one group of people can be held responsible when everything is interrelated.
The World Resources Institute is doing a great job of raising global awareness on eutrophication and has valuable resources online. Locally, the government of Manitoba has been working on developing the Save Lake Winnipeg Act, which can also be accessed online.
The best chance of a solution comes from education and an open dialogue in global and local communities. Eutrophication requires everyone to be proactive where historically we have been blind, and I urge you to get involved in the discussion.





Most of these bluegreen algae do not sink. As the colonies die, part of them lyse, decompose and recycle nutrients as well as releasing toxins into the water . Often they are consumed by amoeba or ciliates and sometimes zooplankton which also help release the nutrients to be used again. There are several species of bluegreen which can produce toxic blooms some times it is a single species and other times it can be several species. They do not all produce the same toxin and there are several different types of toxins that can be produced.
The toxins as well as oxygen depletion can kill fish. There have been fish kills where no oxygen depletion has been recorded but where toxins have been present.
Neurotoxins although less encountered ( thankfully ) are produced by some of our species of bluegreens. They are much more difficult to analyze, and are more deadly and fast acting when encountered then the hepatotoxins typically found and easier to measure. Caution should be exercised when ever a bloom is encountered.